 Hendrik (Henk) Verweij joined the department of Materials Science and Engineering (MSE) at The Ohio State University in March 2001, and was appointed as the Orton Chair in Ceramic Engineering. Before that he was Professor of the Inorganic Materials Science group in the Department of Chemical Engineering of the University of Twente in the Netherlands for 9 years.
Hendrik (Henk) Verweij joined the department of Materials Science and Engineering (MSE) at The Ohio State University in March 2001, and was appointed as the Orton Chair in Ceramic Engineering. Before that he was Professor of the Inorganic Materials Science group in the Department of Chemical Engineering of the University of Twente in the Netherlands for 9 years.
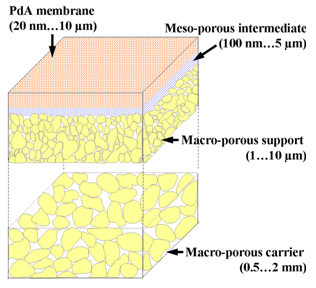
Figure 1: Supported Pd alloy (PdA) membrane structure. In optimized designs the porous layer thickness, X, is roughly proportional to the pore diameter Øp.
Dr. Verweij is currently teaching ceramics processing and has redeveloped several undergraduate labs and reporting classes for the department of materials science and engineering. His research is fully focused on supported inorganic membranes and fuel cells. Membranes as well as fuel cells have the principal advantage that they operate isothermally, which means that their energetic efficiency can approach the reversible thermodynamic limits. Inorganic (ceramic) membranes will play an important role in more efficient separation of O2 from air, CO2 from flue gas for sequestration, H2 from coal gas, and pure water from brackish/contaminated water. Better access to cheap, pure water in the developing world would have a much larger impact than any renewable energy technology. The same applies to large scale energy conversion. The chemical industry uses huge amounts of energy for the separation of C2H4 monomers from C2H4/C2H6 mixtures by cryogenic distillation; the energy requirement in membrane separation would ultimately be limited to pure C2H4 compression work.
Dr. Verweij’s group is currently focusing on fabrication and properties of structures as shown in Figure 1. The membrane’s top layer needs to be very thin, preferably 10…100 nm. The challenge is to make that thin film on a porous support without a single (pinhole) defect. In turn all layers must be homogeneous, dense-packed structures. These structures must be perfect and very cheap to make; parallels must be sought in the semi-conductor industry, rather than in the ceramics industry.
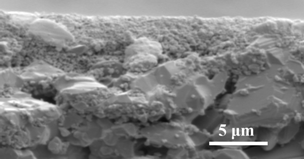
Figure 2: Fracture cross-section of a state-of-the art supported macro-porous membrane. This membrane has has several industrial applications but was not developed for utra-thin gas separation membranes.
For the sake of comparison, a state of the art two-layer structure is shown in Figure 2, and an optimized structure, recently created at OSU in Figure 3. Such structures are now used to deposit 200 nm thin Pd-alloy membranes for H2 separation, nano-composite Pt/(Ce,Gd)-oxide membranes for low temperature O2 separation, zeolites for CO2 separation, and meso-porous TiO2 for water purification.
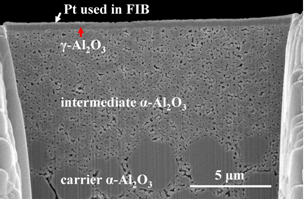
Figure 3: Focused Ion Beam (FIB) cross-section of a three layer membrane support structure.
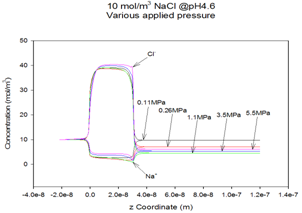
Figure 4: Calculated flow-induced charge-separation in a 4 nm Ø pore channel for a 0.001 M NaCl solution.
Dr. Verweij is also studying characterizing and multi-scale transport properties of supported membrane and fuel cell structures. His transport descriptions are based on concepts from equilibrium as well as non-equilibrium thermodynamics. The transport mechanisms for the subsequent supporting layers vary from ballistic transport and Knudsen diffusion to viscous flow. The membrane layers provide highly selective transport for one molecule by mechanisms that include ambipolar diffusion of charged species, molecular diffusion and space-charge effects as occur in semi-conductor devices. The ambipolar mechanism requires detailed knowledge of the electronic band structure of the membrane materials. Highly selective CO2 separation is based on a strongly correlated multi-component diffusion process for which a theory is yet to be developed. Separation of pure water from a salt solution occurs by “pushing’ it through narrow, open pores. The purification effect is based on a subtle imbalance between ion ion-diffusion, -migration, and –convection. This imbalance is caused by a space charge at the pore entrance, shown in Figure 4, that can develop spontaneously by chemi-sorption of cations on the pore wall. Verweij’s group is also studying thin supported fuel cell structures, not discussed here, using an approach that parallels the membrane work.
The Inorganic Materials Science group recently demonstrated the use of fast colloidal deposition, drying and 600ºC to produce intact membrane layers within one hour. Development of viable membrane and fuel cell concepts and fabrication routes is of extreme importance for a sustainable energy infrastructure and a clean environment. The challenges in fabrication and design are enormous, and require contributions from several scientific and technical disciplines, and Dr. Verweij is eager to co-operate with anyone who can offer new perspectives.
For more information on Dr. Verweij’s research please visit his group webpage at: http://www.matsceng.ohio-state.edu/ims/
